Tit Plif Cage ( Imported Cage ) Exporter

Tit Plif Cage ( Imported Cage ) Manufacturer
Length 20 mm , 25 mm , 30 mm
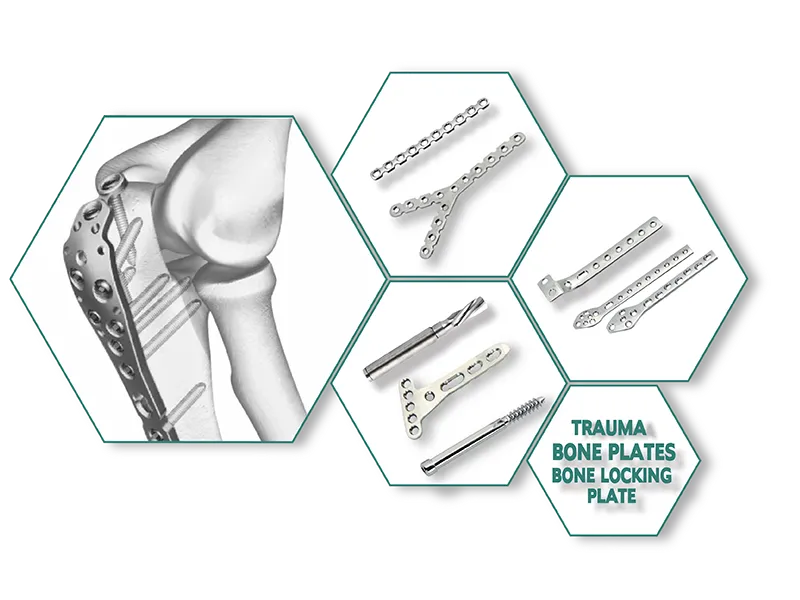
Category:Trauma Implants
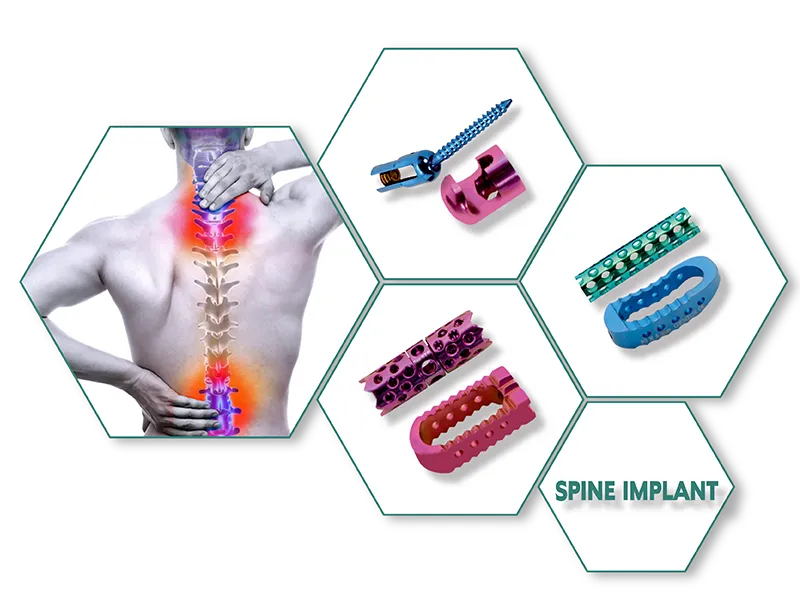
TIT PLIF CAGE (Imported Cage) is a spinal implant device used in posterior lumbar interbody fusion (PLIF) surgery to treat degenerative disc disease, spondylolisthesis, and other spinal conditions. The cage is made of titanium material and has a hollow center that allows for bone graft material to be packed inside, promoting fusion between the vertebrae. It also has a roughened surface to enhance bone ingrowth and stability.
Description
| T.2511.20.07-30.11 | Height 7 mm , 8 mm , 9 mm , 10 mm 11 mm |

Inquiry Now
ABOUT US
Genius Ortho Private Limited
Genius Ortho Private Limited was found in 2008 by a group of entrepreneurs after having a decade of experience in the field of orthopaedic implants manufacturing. Genius Ortho Private Limited now is the leading company in India specialize in the Orthopaedics Industry. Genius Ortho Private Limited will always achieve this position by professional pursuing excellence and by dedicating ourselves in improving the quality and serving all our customers.
All implants are exclusively made of Stainless Steel 316L, LVM & Titanium Grade V Ti-6Al-4v-ELI.
Our products of Orthopaedic implants and instruments are not only qualified with the certification of FDA, CE, GMP regulations, but also compliant with ISO Quality Assurance Standards.