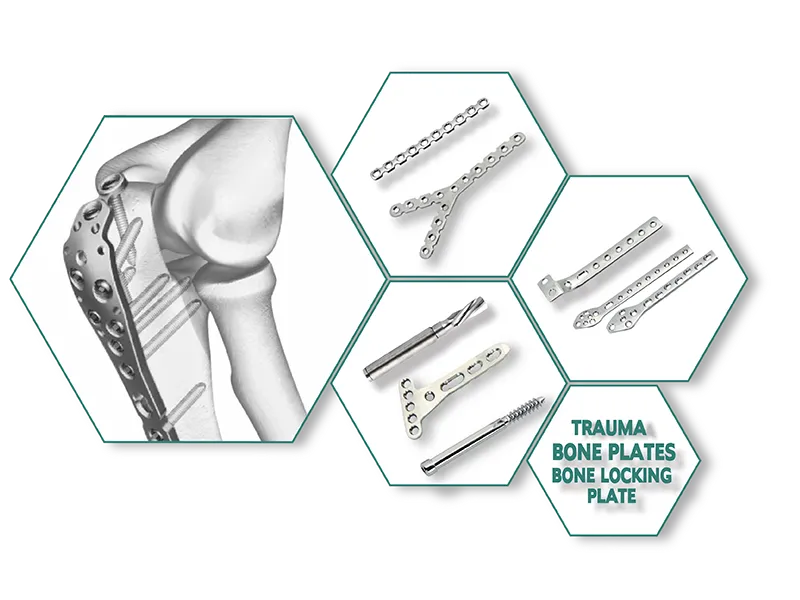
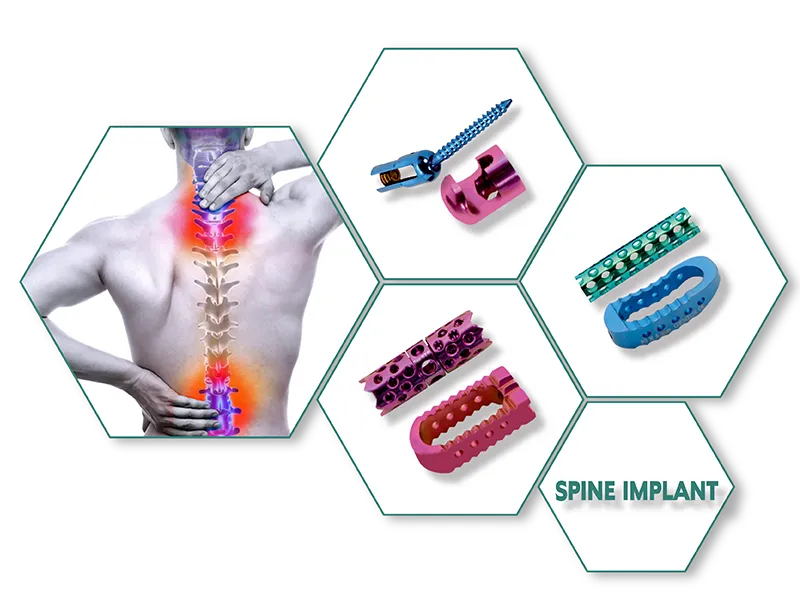
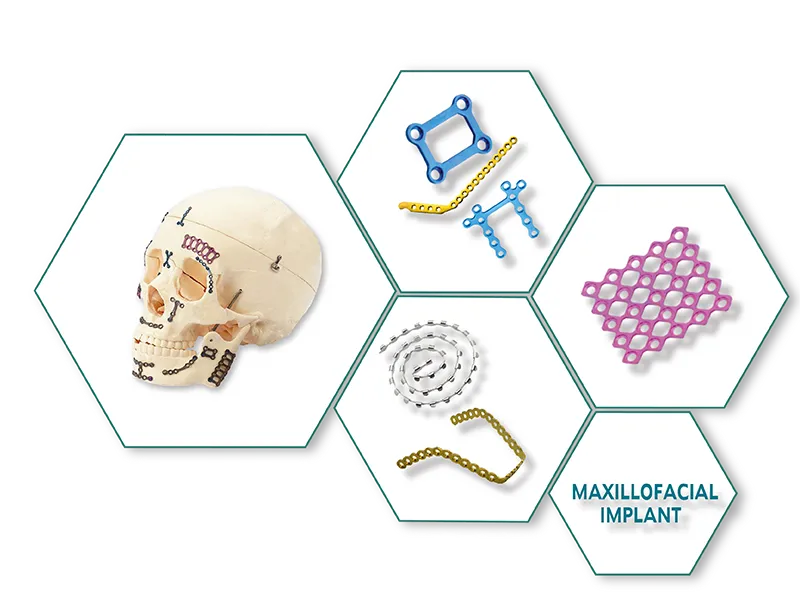
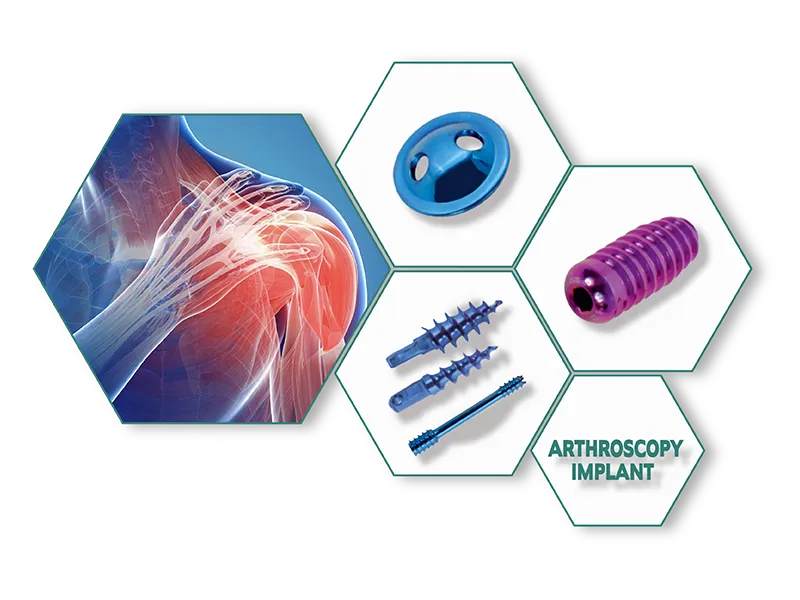
Aesculap Type External Fixator (Radius / Ulna ) Exporter

Aesculap Type External Fixator (Radius / Ulna ) Manufacturer
For Radius / Ulna / Metacarpal
Connection Rod 04 mm
Categories: Aesculap Type External Fixature, External Fixator
Aesculap Type External Fixator (Radius/Ulna) is a medical device used for the treatment of fractures or other conditions affecting the radius or ulna bones in the forearm. The fixator consists of metal rods or wires that are inserted through the skin and into the bone on either side of the fracture. The rods are then connected outside the skin using clamps, which hold the bone fragments in place and promote healing.
Description
| For Radius / Ulna / Metacarpal | |
| S.1102.01 | Aesculap Clamp 2.5 mm X 4.0 mm |
| S.1102.02 | Aesculap Clamp 3.5 mm X 4.0 mm |
| S.1102.03 | Aesculap Clamp 4.0 mm X 4.0 mm |
| Connection Rod 04 mm | |
| S.1102.04.04 | Length 04 Inch (100 MM) |
| S.1102.04.06 | Length 06 Inch (150 MM) |
| S.1102.04.08 | Length 08 Inch (200 MM) |
| S.1102.04.10 | Length 10 Inch (250 MM) |
| S.1102.04.12 | Length 12 Inch (300 MM) |
| S.1102.04.14 | Length 14 Inch (350 MM) |
| S.1102.04.16 | Length 16 Inch (400 MM) |

Inquiry Now
ABOUT US
Genius Ortho Private Limited
Genius Ortho Private Limited was found in 2008 by a group of entrepreneurs after having a decade of experience in the field of orthopaedic implants manufacturing. Genius Ortho Private Limited now is the leading company in India specialize in the Orthopaedics Industry. Genius Ortho Private Limited will always achieve this position by professional pursuing excellence and by dedicating ourselves in improving the quality and serving all our customers.
All implants are exclusively made of Stainless Steel 316L, LVM & Titanium Grade V Ti-6Al-4v-ELI.
Our products of Orthopaedic implants and instruments are not only qualified with the certification of FDA, CE, GMP regulations, but also compliant with ISO Quality Assurance Standards.